Transforming Oncology Drug Development Through AI Insights
Lantern Pharma is leveraging A.I., machine learning & genomics to transform the cost, pace, and timeline of oncology drug discovery and development.
A Pioneering & World Class Team Dedicated to
Creating Novel & Breakthrough Precision Oncology Therapies
"We are in the golden
age of A.I. in medicine."
Key Reasons to Watch
Nasdaq: LTRN
Lantern Pharma is an emerging, oncology-focused, clinical stage pharma at the intersection of Artificial Intelligence, Genomics, and Machine Learning.
Breakthrough Cancer Drug Pipeline: Advancing five clinical-stage drug candidates targeting some of the most difficult-to-treat cancers, with multiple ongoing Phase 1 and 2 trials globally.
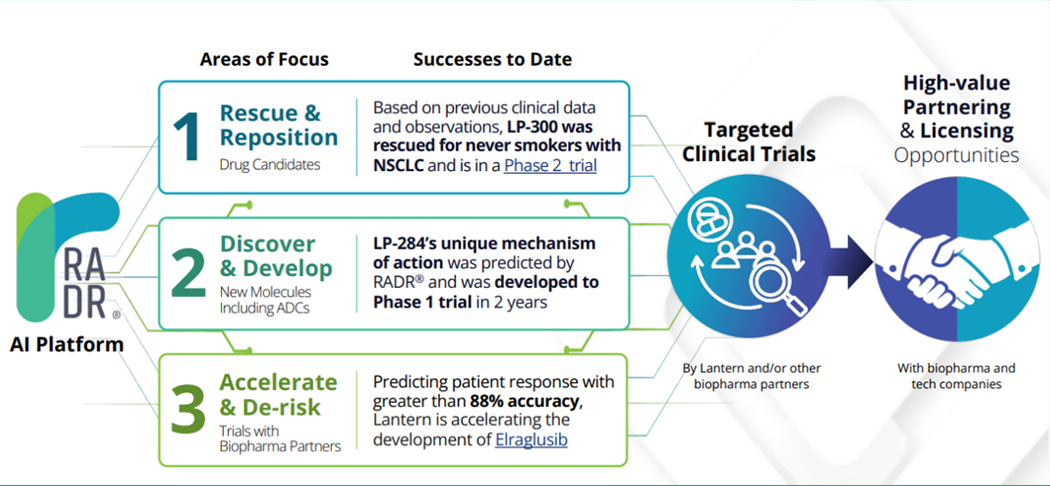
Proprietary AI Platform, RADR®: Lantern’s AI-driven RADR® platform leverages over 100 billion datapoints to accelerate drug discovery by 70% and reduce development costs by 80%, with predictive accuracy of 88% in patient stratification.
FDA Fast Track & Rare Disease Designations: Received multiple FDA designations, including Fast Track for LP-184 in Glioblastoma and Triple Negative Breast Cancer, and rare pediatric disease designations for three childhood cancers.
Strong Clinical Momentum & Global Expansion: Early clinical data shows an 86% clinical benefit rate in the Harmonic™ Phase 2 trial; expanded trials across the US, Taiwan, and Japan with APAC patient enrollment underway.
Strategic Collaborations & Monetization Pathways: Collaborations with leading research institutions like Bielefeld University and efforts to commercialize the RADR® AI platform and Starlight Therapeutics create multiple revenue opportunities beyond drug development.
Fill Out The Form Below To Receive The Most Up-To-Date Information on This Exciting Investment Opportunity.
Who is Lantern Pharma?
Lantern Pharma (Nasdaq:LTRN) is an AI-driven biotechnology company focused on accelerating and optimizing the discovery, development, and commercialization of cancer therapies. Its RADR® platform leverages artificial intelligence and machine learning to uncover novel therapeutic opportunities, accelerate drug development, and improve patient outcomes.
Lantern’s AI platform, RADR, is transforming the cost, pace, and timeline of cancer drug discovery and development
12 Lead drug programs* powered by AI
5 Clinical stage lead drug candidates*
100+ Issued patents and pending applications
$100M Approximate total capital raised since 2019
2.5yrs Avg. time for new LTRN programs for Ph.1 Trial
2M Avg. cost for new LTRN programs for Ph.1 Trial
* Includes drug programs being developed in collaboration
Lantern Pharma’s LP-184 Phase 1a Clinical Trial Achieves All Primary Endpoints with Robust Safety Profile and Promising Antitumor Activity in Multiple Advanced Solid Tumors
September 16, 2025
LP-184 achieves all primary endpoints and demonstrates a favorable safety and tolerability profile positioning it for both monotherapy or synergistic combinations with PARP inhibitors and immunotherapies.
Clinical benefit observed in 48% of evaluable cancer patients at or above the therapeutic dose threshold.
Durable clinical benefits were observed in hard-to-treat tumors like glioblastoma multiforme (GBM), gastrointestinal stromal tumor (GIST) and thymic carcinoma.
Biomarker insights highlight potential in DDR-mutated cancers, with marked tumor reductions in patients with CHK2, ATM, and STK11/KEAP1 alterations.
Recommended Phase 2 dose (RP2D) established for targeted Phase 1b/2 trials in triple-negative breast cancer (TNBC), non-small cell lung cancer (NSCLC), and bladder cancer.
The cancer indications in these targeted trials represent markets exceeding $6 billion in annual potential.
The observations for LP-184 in the Phase 1a trial include clinical benefit for multiple patients who had reached the limits of or failed current available therapies.
DALLAS, September 16, 2025--(BUSINESS WIRE)--Lantern Pharma Inc. (NASDAQ: LTRN), a leading artificial intelligence (AI)-driven oncology company leveraging its proprietary RADR® platform to accelerate targeted cancer therapies, today announced the successful completion of its Phase 1a clinical trial (NCT05933265) for LP-184. The trial met all primary endpoints, demonstrating a favorable safety and pharmacokinetic (PK) profile, and early signs of antitumor activity. Enrollment is complete, with several patients continuing treatment due to ongoing clinical benefit.
The open-label, multicenter, non-randomized study evaluated LP-184 in 63 patients with advanced relapsed or refractory solid tumors, including GBM. Primary objectives focused on safety, tolerability, PK, and determining a recommended Phase 2 dose (RP2D) when administered on Days 1 and 8 of a 21-day cycle.
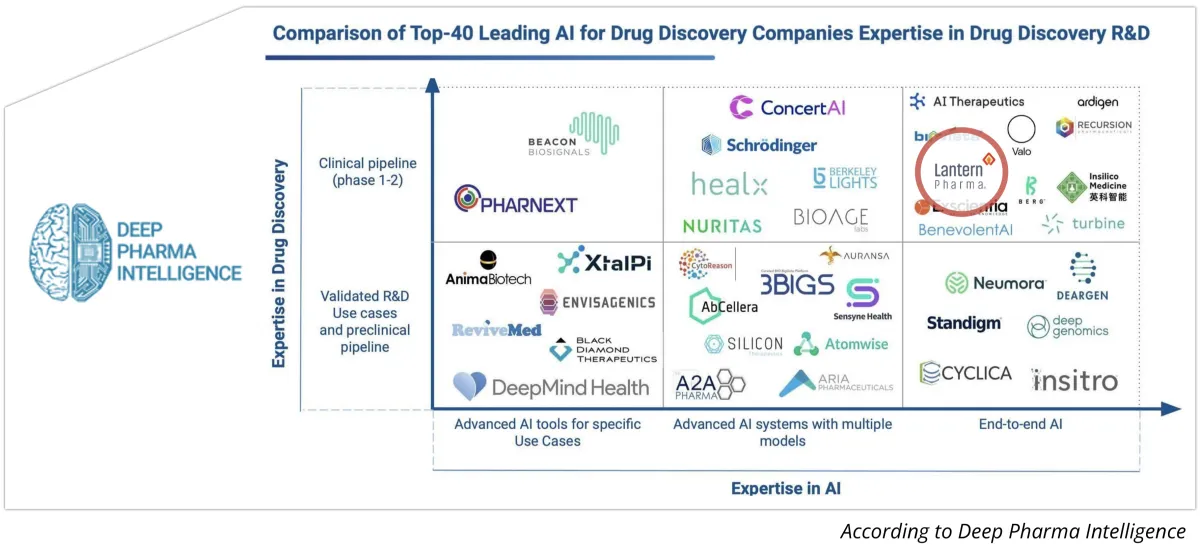
Lantern’s AI-Driven Business Model has Multiple Routes Towards Success
©2025 Lantern Pharma | All Rights Reserved | Privacy Policy | Legal Disclaimer